E Tech Group was involved with an expansion of a Qualified Building Automation System (QBAS) while simultaneously providing the validation documentation and testing in accordance with GAMP 5.0 guidelines.
The QBAS provides alarming, historical data logging, and reporting functionality for the biotech manufacturer. The expansion addressed improvement of the systems reliability, improvement of the user interface, and improvement of system performance.
Integrating the Upgraded QBAS with the SCADA: Project Specs
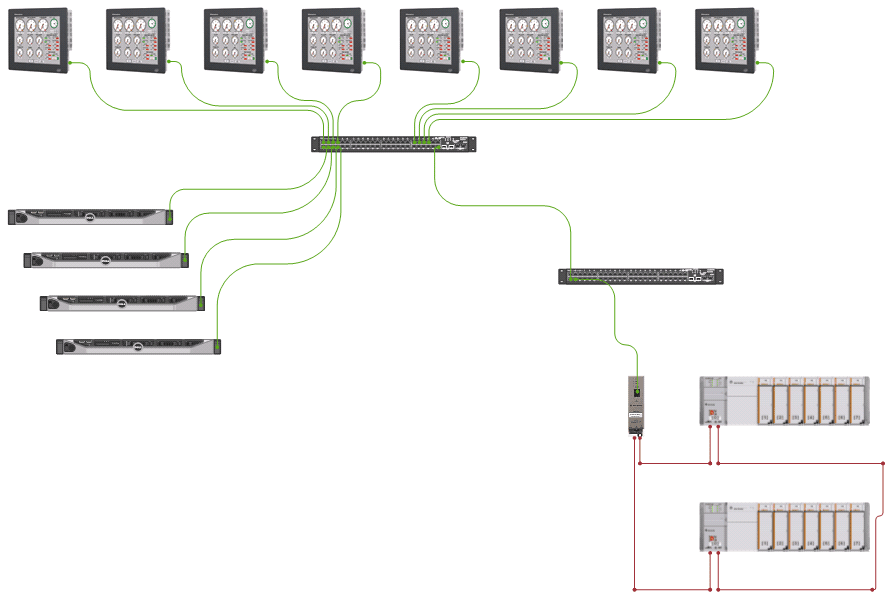
A GE Proficy iFIX SCADA monitors analog and digital signals fed to the process automation controllers (PACs) and third-party skid equipment process automation controllers (PACs). The SCADA logs alarm and events data to a relational database and provides access points for trend and alarm data loggers.
The SCADA hosts the QBAS security configuration. One of the many QBAS clients on the network provide access for QBAS users. Client PCs enable users to monitor system health, monitoring and acknowledgement of process alarms, viewing trends and reports, and saving alarm set points to SQL database.
E Tech’s engineers configured VMWare’s ESX Server to serve as the platform for the QBAS Data Historian and Web HMI virtual servers. The PACs and Client/Server networks operate on different VLANs. The QBAS monitoring PACs are connected in a Device Level Ring topology for high-speed and high-performance all the while providing a resilient process automation controller network for maximum up time.
The SCADA was built on iFIX in Enhanced Failover configuration. Microsoft™ Windows® operating system and rack mounted computers are used as the platform. The computers contain two (2) network interface cards (NIC); one NIC is dedicated to the QBAS communications with PACs and QBAS iFIX clients, and the other NIC for the exchange of Enhanced Failover messages between the redundant pair of SCADA nodes.
The QBAS operates under cGMP so all project lifecycle documentation from URS to IOQ were maintained.
The QBAS was also designed to meet future needs. Strategic grouping of GMP data points, providing additional control panels with I/O and Ethernet communications, and definition of templates with modular scope, all position our customer for a straight forward, time and cost-efficient expansion of their future needs. Focused in biotechnology and the life sciences, E Tech Group has been a trusted systems integrator, providing automation and validation services for over 3 decades. E Tech Group relies on their 140+ systems integration and automation engineers to create custom system solutions for clients in the pharma industry, biotech, and food and beverage industry. E Tech Group also offers comprehensive automation services for the chemical and renewable energy industries.
