The Project: Determine the Scope & Execution of a Control System Upgrade
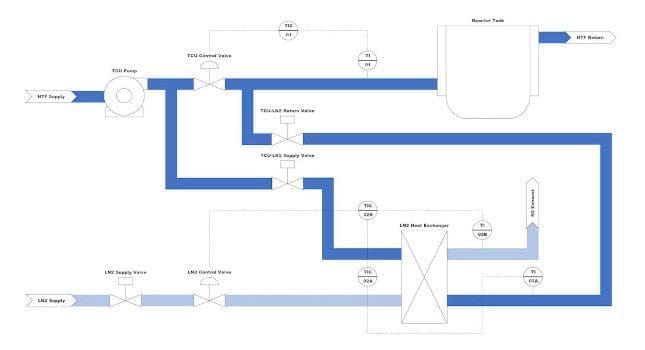
An American biotechnology company was dealing with an aging control system. The vintage installation of GE iFix, iBatch and CompactLogix were approaching end-of-life, making an upgrade essential for multiple locations in California. Understanding intervention by an automation company was necessary.
The client grappled with key questions such as: What is the best control system platform to meet their business needs? Do they upgrade their current system or install a new system altogether? The company approached E Tech Group with this problem, interested in learning about the different platform options and working with a control system integrator to make the right choice based on their needs.
For a project of this magnitude, creating a strong partnership between client and integrator was crucial. Fortunately, the E Tech Group automation team included seasoned engineers who have been through large-scale control system platform selection and conversion projects before and supported similar decisions for other clients.
The Solution: Evaluate, Collaborate, Integrate
E Tech Group went through a process of user requirements definition and user group meetings, assessing the company’s site and global needs. E Tech Group control system engineers participated in workshops with all stakeholders within the company:
- Automation
- Environmental Health &Safety
- Facilities/Maintenance
- Instrumentation
- IT
- Production/Manufacturing
- Process Engineering/Development
- Procurement/Finance
- Quality
- System Integration Partners
Based on experience in providing countless custom controls upgrades, the E Tech Group team challenged the client to think beyond basic requirements from a technical and business perspective. Engaging the team together in workshops enabled each group to learn about how others in the company would be using the system.
At the conclusion of the process, the control platform vendors considered were evaluated against the newly-defined requirements. E Tech Group worked with all of the internal stakeholders at the company to evaluate the options and assess how the different platforms could best support the business’ current and future needs.
Using a scoring system and working with the decision analysis team at the biotechnology company, E Tech Group supported efforts to develop financial and statistical models to help the client determine the best option from a financial and risk perspective.
After deep analysis and evaluation, it was unanimously decided that the proposed DeltaV control system would best help reduce the risk that obsolescence and instability will disrupt the supply of critical patient therapies, help enable agile expansion of future capacity through a thoughtfully-designed automation system, provide flexibility and self-sufficiency for production needs, and ensure delivery of quality product in a compliant manner.
The client and all stakeholders, having been provided with control system software options and educated on the pros and cons of each, reached a unanimous decision on the future platform for the site, landing with DeltaV.
The Result: Long-Term Plans for Long-Term Growth
Understanding what a large financial undertaking this would be, the client, the platform vendor, and E Tech Group continue to collaborate on a long-term roadmap to support the upgrade/conversion of the site to the new platform.